
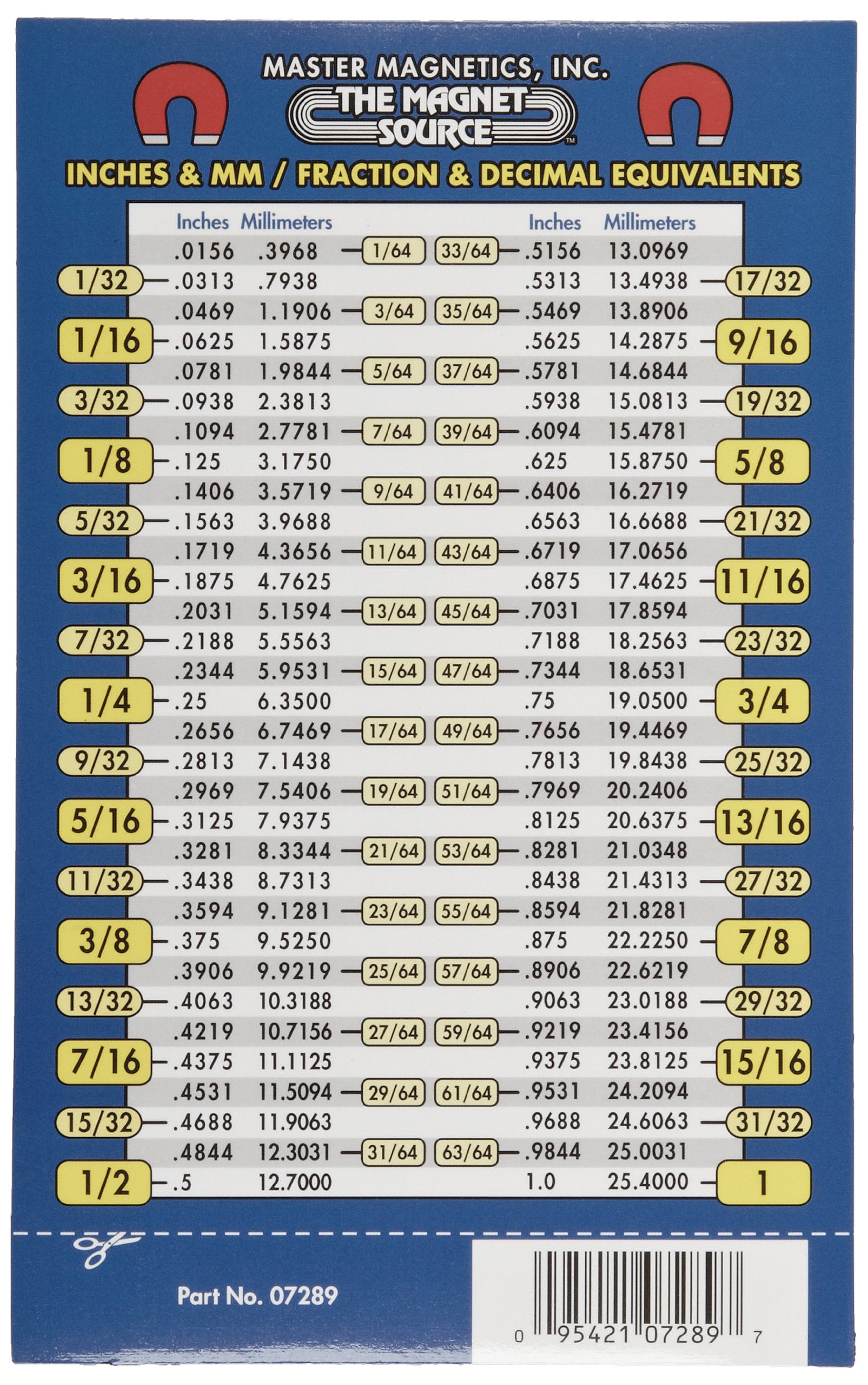

Parts per million (ppm mol) multiply by 100 for mass, mole or volume. Mass per Volume multiply by 100 for mass, mole or volume. Molar Concentration multiply by 100 for mass, mole or volume. Volume Fraction multiply by 100 for mass, mole or volume. The number of moles of KMnO4 will be 0.158Īnd here is how you should enter this problem into the calculator above: grams to moles problem solution Moles to grams example problem Mass Fraction multiply by 100 for mass, mole or volume. Divide the given mass (25.0 g) by the molar mass (158.032 g/mol) to get the moles.The molar mass of KMnO4 is 158.032 g/mol. Problem: Convert 25.0 grams of KMnO4 to moles.įind out the molar mass of the substance (hint: you can use Molar mass of the substance alone to calculate molar mass). Multiplying by the molar mass constant ensures that the calculation is dimensionally correct: standard relative atomic masses are dimensionless quantities (i.e., pure numbers), whereas molar masses have units (in this case, grams/mole).Īnd this is where our grams to moles/moles to grams calculator shines, thanks to our other calculator - Molar mass of the substance, which calculates molar mass for a substance given the formula.Īll you need to do is correctly enter your formula, choose whether you want a conversion from grams to moles or a conversion from moles to grams, and, in case of g to mol, enter the mass, or, in case of mol to g, enter the moles. The molar mass of a compound is given by the sum of the standard atomic weight (namely, the standard relative atomic mass) of the atoms which form the compound multiplied by the molar mass constant. The molar mass of atoms of an element is given by the standard relative atomic mass of the element multiplied by the molar mass constant, 1 × 10−3 kg/mol = 1 g/mol. Greenwashed catalysis The 27th United Nations Climate Change Conference placed the risks of greenwashing under the spotlight. The molar mass is a physical property defined as the mass of a given substance (chemical element or chemical compound) divided by the amount of substance. You need to multiply the molar mass of the substance by the number of moles:Īs you can see, the most difficult task here is finding out the molar mass of the substance.
You need to divide the mass of the substance by the molar mass of the substance:

#Mass fraction to atomic fraction converter how to#
molar mass of the substance in grams/mole How to convert grams to moles? Neither the given number nor the molecular weight that was utilized above are exact numbers.There is a simple relation between grams and moles: Since the math involved in dimensional analysis is multiplication and division, the number of significant figures in each number being multiplied or divided must be counted, and the answer must be limited to the lesser count of significant figures. Finally, the correct number of significant figures should be applied to any calculated quantity. When using a calculator, each conversion factor should be entered in parentheses, or the "=" key should be used after each division. The solution is calculated by multiplying the given number by the value in each numerator, and then dividing by the quantity in each denominator.


 0 kommentar(er)
0 kommentar(er)
